A Pipeline Full of Preventive Cures
The enormous unmet medical need represented by Lumen’s initial targets also demonstrates the size of its addressable markets.
Moving Fast to Save Millions of Lives
Lumen believes that oral biologic drugs are the fastest, safest, best way to generate new treatments. This is particularly true for addressing unintended consequences of antibiotics and improving access to medicines in the developing world, but drug shortages are also a problem in the developed world for highly prevalent diseases like C. difficile infection. Lumen's technology offers new hope to these left-behind patients.
Pipeline
Core pipeline
- Weight loss (LMN-801)Oral hormone proteinMOA Previously DemonstratedPreclinicalPhase 1
(safety)Phase 2
(proof of concept)Phase 3
(definitive efficacy)Weight Loss
Product Candidate
Mode of action:Oral hormone protein
Phase 2
A significant portion of the global population is overweight with rising prevalence and limited effective, safe, and scalable treatment options. LMN-801 is designed as a potential first-in-class, orally delivered biologic (a hormone protein) that targets metabolic signaling pathways to promote fat loss while preserving lean muscle mass—addressing key drawbacks of existing injectable therapies that often cause muscle wasting. If successful, it could fulfill the unmet need for a highly accessible, globally scalable treatment for overweight individuals. - C. difficile (LMN-201)Oral biologic cocktailMOA Previously DemonstratedPreclinicalPhase 1
(safety)Phase 2
(proof of concept)Phase 3
(definitive efficacy)

C. difficile Colitis
(Clostridioides difficile)
Product Candidate
Mode of action:Oral biologic cocktail
Phase 2/3
Traditionally considered a hospital-acquired infection, C. difficile is more commonly community-acquired today. Long-term consequences include chronic diarrhea, severe intestinal inflammation, surgical resection, and death. Insidiously, the very thing that is used to treat this infection—broad spectrum antibiotics—is also the thing that causes the disease. C. difficile appears near the top of the CDC’s antimicrobial threats list year after year. For these reasons, the orally delivered LMN-201 will be an important potential addition to the struggle against antibiotic-resistant infections. - IBD (Crohn’s/UC)Oral biologic cocktailMOA Previously DemonstratedPreclinicalPhase 1
(safety)Phase 2
(proof of concept)Phase 3
(definitive efficacy)IBD
(Crohn's disease and ulcerative colitis)
Product Candidate
Mode of action:Biologic cocktail
Preclinical
Inflammatory bowel disease (IBD) is a systemic autoimmune disease, but it has an obvious nexus with the gastrointestinal tract. The two main sub-varieties—Crohn’s disease and ulcerative colitis—have many available treatments on the market today, but it remains a major unmet medical need: current treatments are costly, require inconvenient injection delivery, and often come with severe side effects. There are no approved advanced therapies at all for mild-to-moderate IBD. A safe, affordable, orally delivered drug to root out IBD at its source is a straightforward application of Lumen's technology, and would constitute a major advance for the field. - Oxalate malabsorptionEnzymeMOA Previously DemonstratedPreclinicalPhase 1
(safety)Phase 2
(proof of concept)Phase 3
(definitive efficacy)

Oxalate degradation
Product Candidate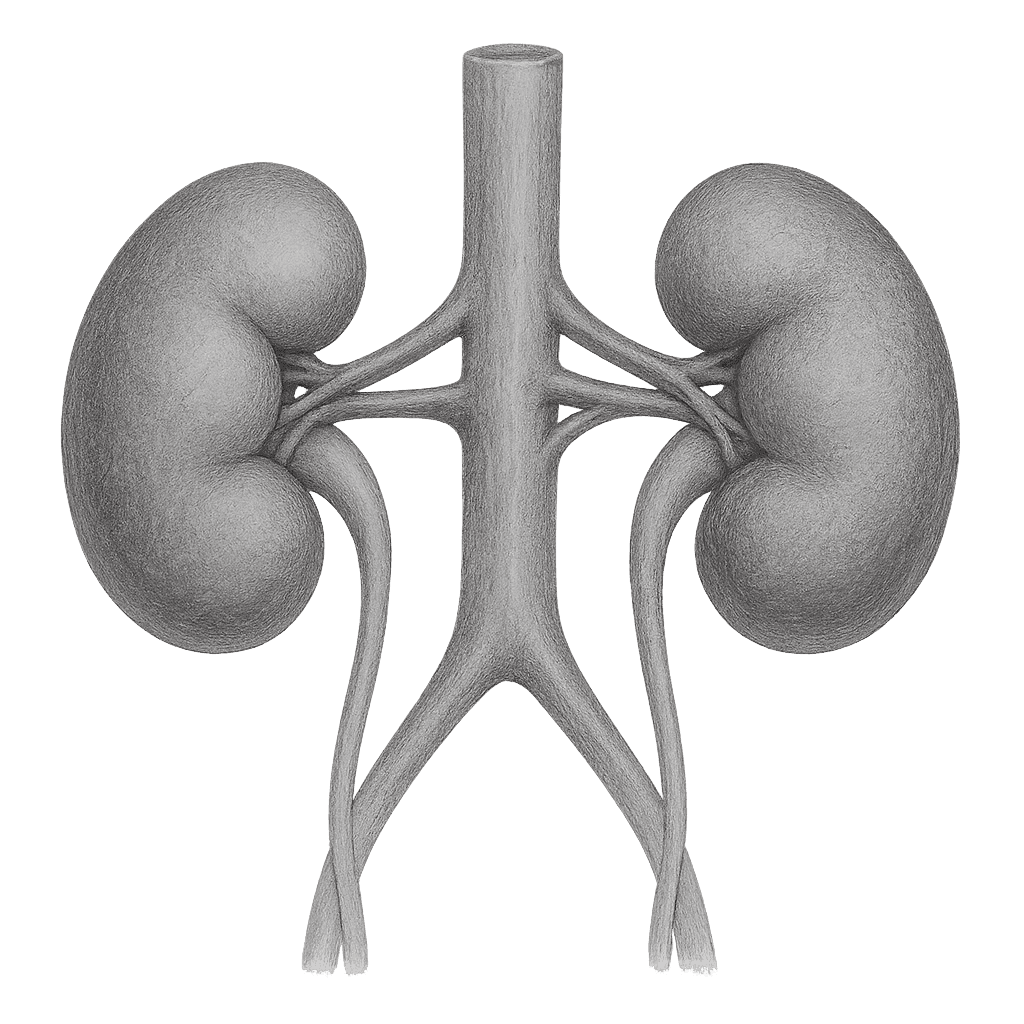
Mode of action:Enzyme dietary supplement
Phase 2/3 ready
Kidney stones affect millions worldwide, leading to discomfort, frequent recurrence, and costly medical procedures. Current preventive approaches are limited. Lumen’s novel oral biologic supplement is designed to support kidney and urinary tract health by helping maintain healthy metabolic balance and normal crystallization pathways. By promoting a favorable urinary environment, it may help reduce the risk of recurring stone formation. If validated through ongoing studies, this product could represent the first non-invasive, biologic-based nutritional approach for supporting long-term kidney wellness.
Innovation pipeline
- GastroenteritisAntibody cocktailMOA Previously DemonstratedPreclinicalPhase 1
(safety)Phase 2
(proof of concept)Phase 3
(definitive efficacy)

Gastroenteritis
(Various Etiologies)
Product Candidate
Mode of action:Antibody cocktail
Field POC study planned 2026
Acute gastroenteritis, caused by viral or bacterial pathogens, is a major global burden—especially in children and low-resource settings—leading to dehydration, hospitalizations, and even death. Lumen’s antibody cocktail is designed to neutralize the most common pathogens in the GI tract. Early field and ileostomy pharmacokinetic studies have validated delivery and local action; a proof-of-concept study in 2026 will assess effectiveness in real-world scenarios. - Viral Respiratory InfectionsIntranasal biologicMOA Previously DemonstratedPreclinicalPhase 1
(safety)Phase 2
(proof of concept)Phase 3
(definitive efficacy)
Viral Respiratory Infections
(Influenza, SARS-CoV-2, etc.)
Product Candidate
Mode of action:Intranasal biologic
Phase 1
Viral exposure is a constant challenge, especially in shared environments where transmission risk is high. Traditional preventive measures are limited, and many people are looking for safe, non-invasive options to support their body’s first line of defense. Lumen’s novel intranasal biologic supplement is designed to help support the nasal passages—the body’s natural gateway—by maintaining a healthy protective barrier and promoting an environment unfavorable to viral attachment. By supporting localized immune balance and helping block environmental challenges at the point of entry, it may reduce the likelihood of person-to-person spread. If validated in ongoing research, this approach could represent a first-of-its-kind biologic-based supplement to support everyday immune resilience and transmission protection. - MalariaIntranasal vaccineMOA Previously DemonstratedPreclinicalPhase 1
(safety)Phase 2
(proof of concept)Phase 3
(definitive efficacy)
Malaria
(Plasmodium infection)
Product Candidate
Mode of action:Intranasal vaccine
Exploratory/Preclinical
Malaria remains one of the world’s deadliest infectious diseases, with hundreds of millions of cases annually and rising resistance to frontline treatments and insecticides. Despite progress in vaccine development, coverage and durability remain limited, and affordable interventions are still urgently needed. If successful, this could provide a low-cost, stable, and easily distributed adjunct therapy for endemic regions—helping fill a critical gap in malaria control strategies.