Step 1: The Organism
Because spirulina cells are extraordinarily high in soluble protein (>60%), they can express much higher amounts of therapeutic proteins than any other food crop.
Lumen unlocks the full potential of biologic drugs, whose promise has been held back by the lack of scalable technology. Our goal is to transform the way such drugs are developed, what they cost, and who has access to them.
Lumen's patented technology allows us to use the well-known food algae spirulina to deliver therapeutic proteins. It is also useful for important applications in human and animal nutrition.

Our patented technology allows us to develop biologics faster with lower cost and greater potential for risk reduction than traditional methods.
Because spirulina cells are extraordinarily high in soluble protein (>60%), they can express much higher amounts of therapeutic proteins than any other food crop.
A gene encoding the therapeutic molecule (for example, an enzyme, antibody protein, or cytokine) is introduced into the spirulina chromosome.
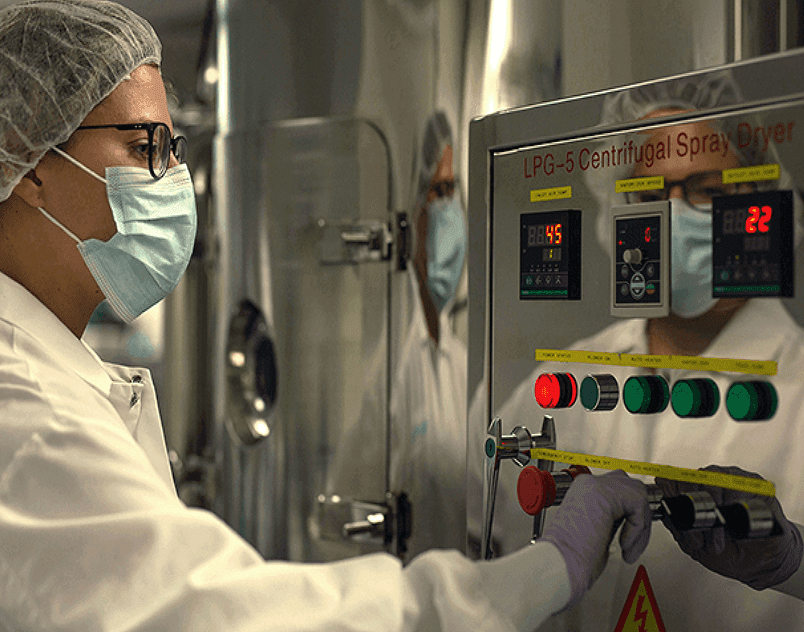
The production system requires only water, salt, CO₂, and light, so it is inexpensive and highly scalable. The entire biomass is simply spray-dried to a fine powder.
This powder can be packed into dose-specific capsules. Most are shelf-stable at room temperature.
Traditional drug development has failed to address a broad swath of high-burden diseases—not because the need isn’t there, but because the tools weren’t built for the job. This has left massive markets hiding in plain sight. Lumen’s unique oral biologics platform is purpose-built to unlock these opportunities with speed, precision, and affordability—disrupting entrenched models and redefining what’s possible in modern drug development.
Our lead program is LMN-801, a potential first-in-class oral product for weight loss
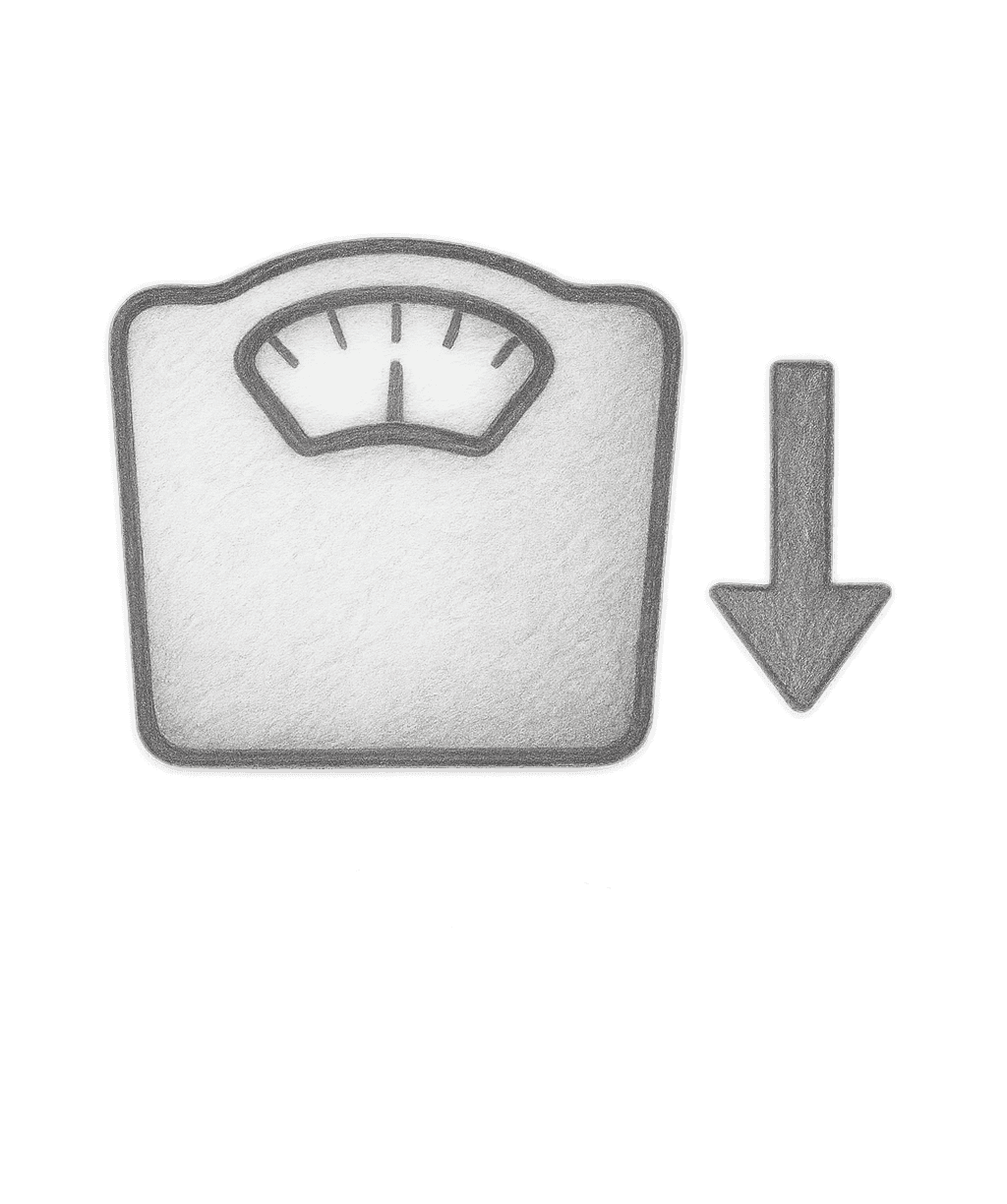
Mode of action:Oral hormone protein
Phase 2


(Clostridioides difficile)
Mode of action:Oral biologic cocktail
Phase 2/3
(Crohn's disease and ulcerative colitis)
Mode of action:Biologic cocktail
Preclinical


Mode of action:Enzyme dietary supplement
Phase 2/3 ready

(Various Etiologies)
Mode of action:Antibody cocktail
Field POC study planned 2026

(Influenza, SARS-CoV-2, etc.)
Mode of action:Intranasal biologic
Phase 1

(Plasmodium infection)
Mode of action:Intranasal vaccine
Exploratory/Preclinical
Collaboration has helped get us to where we are today, and it is key to addressing the full range of opportunities that exist for mass-scale biologics.